Home » Properties of Colourants
Properties of Colourants
Inorganic pigments, organic pigments and dyes
Organic and Inorganic Pigments
Inorganic pigments are usually insoluble metal salts or metal oxides whilst organic colorants generally derive from the petrochemical industry and are characterized by carbon to carbon bonds in the structure.
When comparing the two classes inorganics are generally more opaque and non-migratory. They exhibit very good heat stability and light fastness properties.
Organics show considerably more tinting strength, often coupled with higher price per kg. They are generally brighter in reduction (but not always in full shade) than inorganics. They are generally poorer in heat and light fastness and being slightly soluble have a tendency to migrate.
In recent years the heavy metal content of some inorganic pigments has caused them to come under close scrutiny for toxicological reasons.
I will describe colourant types finding significant application in plastics. They have been grouped according to their chemical composition. Each group is briefly described in terms of their composition colours obtainable properties and applications.
Dyes
These are organic colourants which dissolve in the application medium. They are only used to colour plastics that are polar and “glassy” rather than crystalline e.g. polystyrene, acrylic, etc. i.e. having a high Glass Transition temperature (Tg). In polyolefins, where the service temperature is above Tg, dyes exhibit poor resistance to migration.
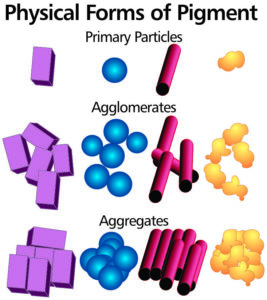
Nature of pigment particles
A sample of pigment contains primary particles, aggregates and agglomerates.
Primary particles or crystallites are the ultimate form in which a pigment exists. Depending on the type of pigment these may be of cubic, spherical, rod-like, flake or irregular form. Because of their high surface energy these primary particles readily form larger clusters called aggregates and agglomerates.
Aggregates consist of firmly bound crystallites joined together at their faces.
Agglomerates result when primary particles join at corners and edges in a loosely bound arrangement.
Aggregates also contribute to the formation of large agglomerates, and in practice the transition between primary particles, aggregates and agglomerates is not well defined. When dispersing a pigment, agglomerates can be extensively broken down into smaller particles, but generally it is very difficult to divide aggregates.
The dispersion process
Dispersion occurs when the initial separate masses of pigment and plastic are changed into one homogeneous mass, with the pigment uniformly distributed throughout the plastic.
Four things happen during the dispersion process. The pigment particles are separated and each is thoroughly enveloped or wetted-out with the molten polymer. Agglomerates are broken down as completely as possible to their components, and all particles are distributed uniformly throughout the polymer mass. I have expanded on dispersion in the section called ‘Preparation of concentrates’.
Factors affecting the dispersibility of pigments
Type of pigment
The type of pigment, the nature and wettability of the pigment surface, the binding forces acting between the particles and the type of plastic to be coloured, all affect dispersion.
A pigment for plastic should have these desirable properties –
- Minimum of agglomerates
- Low agglomerate strength
- Ability to be rapidly wetted out by the molten polymer
Nature of the pigment
Pigment particles have a tendency to re-agglomerate under compressive pressure, such as occurs during blending and in the feed zone of an extruder or injection moulder. These large, hard agglomerates, which were not originally present in the dry pigment sample, are sometimes called comprimates.
In general this tendency to ball together varies from one type of pigment to another but is more marked with the softer, lower melting organic pigments, than with inorganic pigments which have high melting points and hard individual crystallites.
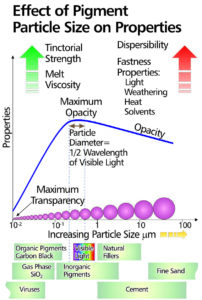
The size of the primary particles has a large effect on pigment properties, including dispersibility which improves with increasing particle size.
Small primary particles, because of their high surface energy, form more stable agglomerates than do large particles. Fastness properties and melt rheology also improve with increasing particle size.
To counteract the above advantages tinctorial strength decreases strongly with increasing particle size, so it is necessary to arrive at a compromise. Whenever possible the manufacturing process is controlled in such a way that the pigment is obtained in a particle size optimally suited to its main use.
Inorganic pigments are usually ground to between 0.2 and 0.35 micron to optimise covering power, since for maximum opacity, the particle size should be of approximately the same magnitude as half the wavelength of visible light.
Apart from some exceptions organics usually possess a particle size of less than 0.1 micron and so are more or less transparent.
Particle size distribution
Pigment particles are not identical but vary to some extent from a mean value.
Generally they narrower the size distribution the better the performance with less likelihood of advantages gained in one respect being offset by the poorer properties in other respects.
Surface treatment
Good dispersibility and other advantageous properties, can also be achieved by developing the small primary particles with surface active substances. The strong surface forces, in particular the energy rich lattice defects, are screened off and agglomeration is thus largely prevented.
Pigments intended for plastics applications often receive a surface treatment to improve wettability with polymer melts.
© Vibron 2018