Home » Organic Pigments for Plastics
Organic Pigments for Plastics
Organic pigments used in plastics can be classified into three major categories: azo pigments, polycyclic pigments and carbon black.
Manufacture of organic pigments
Most organic pigments are manufactured from a few basic raw materials such as the aromatic hydrocarbons available from coal tar or petroleum distillates.
Using standard reactions of organic chemistry, these are first converted into a variety of intermediates. Selected intermediates are then combined to form a very wide range of colour pigments and dyestuffs.
Azo pigments
These are the classical organic pigments and contain one or two azo groups (-N=N-) as chromophores. Groups which give rise to colour are called chromophores, whilst those which intensify or modify the colour are called auxochromes. By altering the molecular structure yellows, oranges, reds, bordeaux, violets and browns are produced.
This family can be further subdivided into monoazo, disazo and disazo condensation pigments. Their general properties can be described, keeping in mind that every pigment is an individual. Wide property variations are encountered within any group.
Monoazo pigments
Many of the simpler monoazo pigments display very good light fastness, particularly in full shade. However they tend to dissolve in polymer melts, solvents and plasticizers. Their migration resistance and heatstability are generally inadequate for use in plastics.
Chemical solubility can be reduced by increasing the molecular size and or introducing polar groups; thus producing monoazo pigments which can be used to colour plastics.
Laked monoazo pigments, or toners
These are metal salts of monoazo dyes containing carboxyl or sulpho groups. Their pigment properties and good migration resistance derive from the polar salt-like structure. Light and weathering fastness are generally poor, particularly in tints. Many are bright and low in cost, so are widely used in PVC, rubber, styrene and polyolefins in non-critical applications, if the processing temperatures are not too high.
Improved monoazo pigments
Monoazo pigments with good to excellent lightfastness and substantially improved heatstability and migration resistance, can be obtained by enlarging the molecule and incorporating polar groups such as carbonamide (CO-NR2) and benzimidazolone (-NH-CO-NH-). These Improved monoazo pigments are used in plastics processed at 260ºC, and in some instances even higher, wherever their moderate-to-high price permits.
Disazo pigments
This class of pigments contains two azo groups as the chromophore. Compared to their monoazo counterparts, disazo pigments are generally faster to migration and tinctorially stronger, but are inferior in light fastness. Their very high tinctorial strength and relatively low cost made some disazo pigments attractive for the colouration of plastics. Unfortunately diaryl pigments are generally the most useful members of this group. These should not be used in polymers if the processing temperature exceeds 200ºC because of possible thermal decomposition, which can form traces of aromatic amines. They can be used in applications like cables and extruded film if the processing temperature is below 200ºC.
Disazo condensation pigments
Introduced in the early 1950’s by Ciba-Geigy, azo condensation pigments, whose molecular weight can be over 1000, represent a further improvement in the range of azo pigments. Although they are expensive, the more heatstable ones are often used to obtain “organic” formulations to colour injection-moulded polyolefins and styrenics.
Polycyclic pigments
For further improvement in resistance to high temperatures, we must look among the polycyclic class of substances rather than among the azo pigments.
Phthalocyanine pigments
These are the most important class of organic pigments for plastics. Most commonly used in plastics are the reddish, stabilized α form and the greenish β form of copper phthalocyanine blue; as well as the polychlorinated and polybrominated greens, which are even more lightfast and heatstable than the blues.
Phthalocyanine pigments are low in colouring cost, very bright, display very good to excellent heat stability and other fastness properties, so they are very widely used in plastics. Their major drawback is a tendency to nucleate some resins e.g. HDPE.
Quinacridone pigments
These range in shade from a dull golden yellow to bright bluish reds and violets. They perform on par with phthalocyanines in PVC and polyolefins but partly dissolve and react in polar plastics e.g ABS and acrylic. Although relatively expensive the reds and violets are widely used, particularly as shading components.
Dioxazine violets
These show excellent light fastness and extremely high tint strength coupled with moderate heat stability and migration resistance. Used in injection mouldings when a bright violet shade is required. Dioxazine violet has an unfortunate tendency to partly dissolve in polymer melts. It then re-crystallizes on cooling, in its monomolecular form, which is a fluorescent pink. For this reason it should not be used at low concentrations.
Isoindolinone Pigments
These range from greenish-yellow to orange in shade and display very good fastness properties. Heat stability and colouring costs are similar to that of the disazo condensation pigments.
Perylene and Perinone pigments
Perylenes range from bright scarlet to maroon, whilst a bright orange and a dullish red are produced from perinone. Perylene red and perinone orange exhibit excellent all around properties but are very expensive. Like dioxazine violet, perinone orange also has a tendency to partly dissolve in polymer melts and re-crystalize on cooling in its monomolecular form. In its case the monomolecular form is a fluorescent reddish yellow, so low concentrations are best avoided.
Thioindigo Violet
This is sometimes used as a slightly cheaper replacement for quinacridone violet but its heat stability is only 260°C, which limits its usefulness in injection moulding applications.
Anthraquinone pigments
These were developed from vat dyes and contain a carbonyl group as the chromophore. Many show excellent fastness properties but are rarely used to colour plastics because of their high price. Best known examples of this group are flavanthrone yellow and indanthrone blue.
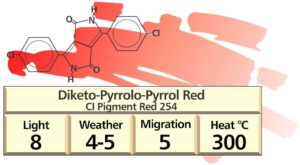
Diketopyrrolopyrrole (DPP) pigments
The DPP pigment is the only truly new chemical pigment family commercialised during the past 50 years.
It was first synthesized in 1974 by a Michigan State University chemistry professor, as the product of a failed experiment aimed at creating a different compound. Someone at Ciba-Geigy found his chemical description of the material and realized its’ potential as a new class of pigment.
The pigments display similar, excellent top-tone brightness to cadmium reds. The heat-stability and all round fastness properties are in a similar class to the phthalocyanine and quinacridone pigments. Thus finally in the ‘80s, a mid-shade red became available, which could function as a viable cadmium pigment replacement in many colour matchings. When the patents expired, Chinese equivalents became available at roughly 25% of the original price; so it also became it very competitively priced against cadmium reds.
Soluble Dyes
These are exclusively organic compounds and can be oil soluble azo or polycyclic compounds or metal complexes. The colour range covers the full spectrum. Fluorescent colours and optical brightners also belong to this class. Fluorescent pigments are manufactured from fluorescent dyes by dissolving the dye in a suitable resin, which is then ground to a pigment particle size.
Being soluble they have a strong tendency to migrate out of a plastic that contains mobile amorphous regions. For this reason they should only be used in polymers with a high Tg (glass transition temperature) such as polystyrene, acrylic, polycarbonate; where they often display very good all round properties.
Carbon black
Composed of finally divided carbon, carbon black is sometimes classified with inorganic and sometimes with organic pigments. It has excellent tinctorial strength and hiding power. The heat stability, light and weather fastness of carbon black are all very good. In addition it is a very effective UV absorber, often incorporated to protect the polymer from UV radiation.
Carbon black is generally inert, and when free from impurities, is nontoxic. Because of its very small particle size it is difficult to optimally disperse in plastics. It also has a strong tendency to dust and cause contamination. For the above reasons it usually incorporated into plastics in the form of a pre-dispersed concentrate.
There are several different types of carbon black available, classified according to the method of manufacture e.g. furnace black, channel black, lamp black, bone black, etc. Furnace black is by far the most popular.
Applications: Due to its excellent all round properties and its very low price, carbon black is very widely used in plastics as a colourant, UV stabiliser e.g. in polyethylene pipe, and a reinforcing filler e.g. in car tyres. Because of its excellent hiding power it is commonly used to colour recycled material. Carbon black is the second most widely used plastics colourant after titanium dioxide white, and accounts for about 20% of all polymer colourant usage by weight.
© Vibron 2018