Home » Inorganic Pigments for Plastics
Inorganic Pigments for Plastics
Click on any image to cycle through the images in this section
The table illustrates the inorganic pigment families that are used in plastic. They are metal oxides, mixed oxides, sulphides, lead chromates and lead molybdates.
Certain types that do not fit into the above categories are also used for obtaining special effects.
Manufacture of inorganic pigments
Synthetically produced inorganic pigments usually employ one or more of the following preparation methods. In each case the end product is washed, filtered, dried and ground –
- Precipitation: This is a reaction of two or more water soluble metal salt solutions, which when mixed, produce an insoluble coloured precipitate
- Precipitation followed by calcination: The precipitated metal salt is washed dried and then roasted at an elevated temperature to bring out the true pigmentary properties
- Calcination: The components are intensively blended then heated to around 1,000ºC. The heat energy supplied to the system initiates a solid-state reaction resulting in a coloured single phase.
As a general rule pigments which undergo calcination during manufacture are more inert and heat stable than those which are simply precipitated.
Metal oxides
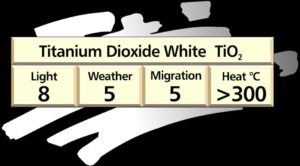
Titanium dioxide TiO2
This is the most widely used plastic colourant and accounts for about two thirds of all colourant usage by weight. Its excellent all round properties make it the preferred white for almost all polymers and processes. The rutile grade of titanium dioxide is preferred to anatase which can cause chalking on weathering.
Today very little use is made of the older whites due to their inferior cost-performance characteristics.
Plastics grades of titanium dioxide are usually enveloped in a composite coating of alumina silica and siloxane to improve weatherability, dispersibility and minimize moisture pickup.
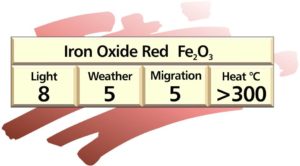
Iron oxide pigments
These pigments are available as naturally occurring “Earth colours” and synthetic forms.
The synthetic oxides are used in plastics because of their better colour strength, purity, reproducibility and uniform chemical composition.
Synthetic iron oxides are manufactured in a variety of relatively dull reds, yellows, browns and black iron oxide. Iron oxide red α-Fe2O3 is stable to 1200ºC. Iron oxide yellow, brown and black are stable to about 180ºC and are eventually converted to the red α-Fe2O3. Brown and black iron oxide based pigments with similar heat stability to the red iron oxide can be produced by partly replacing the iron atoms in the iron oxide lattice structure.
Properties of iron oxide pigments: these pigments range in shade from yellow/buff to terracotta to browns and blacks. They feature excellent hiding power and tint strength. Colour is influenced by composition, crystal structure, and particle shape and particle size. For example by varying the particle size between 0.1 and 1.0 microns opaque iron oxide reds of very different shades can be obtained.
If the particle size is reduced to about 0.01 micron transparent iron oxides are obtained. Iron oxide reds, and modified heat-stable browns and blacks exhibit excellent heat stability in all polymers. The conventional yellows, blacks and browns are only heat stable to about 180°C which limits their use in plastics. All types of iron oxide pigments exhibit excellent light and weather fastness. Iron oxides are fast to most chemicals and solvents.
The iron in these pigments can degrade some resins. This is a problem only under extreme conditions of service. A concentrate that contains a lot of iron oxide should also contain extra antioxidant to protect the polymer during processing. The opaque types are usually easy to disperse but the transparent iron oxides, due to their high surface area, have all the dispersion and rheology problems normally associated with organic pigments.
This class of pigments is generally regarded as being completely safe to use in food contact and children’s toys applications and these pigments are amongst the cheapest plastics colourants available today.
Applications: The synthetic reds and high heat stable brown and black oxide are very widely used in plastics because of their low cost coupled with excellent fastness to light, heat, chemicals and solvents. Only their limited heat stability prevents the yellows and conventional browns and blacks from being equally popular but these find outlets in polymers requiring low temperature processing, such as PVC and polyester casting resin.
Chrome Oxide Green
Chrome oxide green is an opaque, dull, almost olive, green exhibiting excellent heat stability, light and weather fastness, coupled with chemical inertness. It is made by calcining sodium and potassium dichromate under reducing conditions followed by washing, drying and grinding.
Chrome oxide green is used if colouristically feasible wherever its excellent stability, inertness in polymers and chemical resistance is required.
Mixed Oxide Pigments
These pigments are a direct product of high temperature solid-state reactions. They are obtained by roasting an intimate mixture of the corresponding oxides, hydroxides and salts, usually at over 1000°C.
When sufficient heat energy is supplied into the system, as diffusion of ions take place, resulting in a homogeneous coloured pigment.
There are very many mixed oxide pigments in existence, often initially developed for the ceramics, glass and vitreous enamel industries. So far only a few have found significant application in plastics.
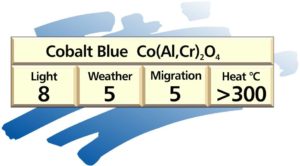
Cobalt blue
They are usually mixed oxides of cobalt and aluminium, silicon, zinc and chrome. Chrome changes the shade from royal blue to turquoise.
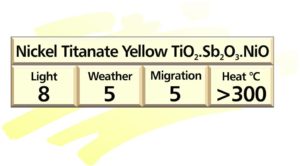
Titanium pigments
These pigments all display structure similar to the rutile modification of titanium dioxide, with some of the titanium replaced by various chromophores. Nickel titanate yellow is the best known example of this type and is a mixed oxide of nickel antimony and titanium – TiO2.Sb2O3.NiO.
Another type used in plastics is chrome antimony titanium yellow – TiO2.Sb2O3.Cr2O3.
Cobalt green, sometimes called titanate green, also comes under this category. It is usually a mixed oxide of titanium, nickel and cobalt, often with aluminium, zinc or magnesium incorporated into the crystal lattice – typically TiO2.NiO.ZnO.CoO.
Mineral blacks
Co-calcined mixtures of various oxides e.g. Fe2O3.Cr2O3.CuO, Fe2O3.MnO2, CuO.Cr2O3.MnO2
Zinc Iron Yellow
A mixed oxide of zinc and iron with a spinel structure ZnO.Fe2O3. The shade is between iron oxide yellow and iron oxide red. It is lightfast and heatstable in plastics.
YInMn Blue
A mixed oxide of Yttrium (Y), Indium (In) and Manganese (Mn). It has been recently introduced commercially at a stratospheric price, and is likely to remain expensive because of its high priced raw materials. Similar in shade to Cobalt and Ultramarine blues, it is unlikely to become a mainstream part of the plastics colourist’s palette.
Bismuth Vanadate Yellow - BiVO4
Manufactured in greenish to reddish yellow shades, it was first mentioned in a 1924 patent but has not been commercialised till the 1980s. At that time, lead chromates and cadmium sulphides came under increased scrutiny for toxicological reasons. Bismuth vanadates, at roughly 10 times the cost of lead chromates, suddenly found an ecological niche as heavy metal replacement pigments. It is difficult to replace full-shade lead chromates with high performance organic yellows as these tend to lack the full-shade brightness and opacity, and are generally also very expensive.
Bismuth vanadates have excellent all-round properties and are widely used in automotive and architectural coatings. In contrast to other members of the mixed oxide pigment family, the heat stability of the standard grades is only about 220°C. This limits their usefulness in plastics, but silica coated grades, stable to 300°C are available.
Properties of mixed oxide pigments
This type of pigment tends to be less bright and lower in tinctorial strength than most other pigments used in the plastics industry, consequently colouring costs tend to be moderate-to-high.
The heat stability and all fastness properties are excellent usually well in excess of demands placed upon them by the plastics industry. Typically they are heatstable to 1000ºC and suitable for 20 years of outdoor exposure.
The mixed oxide pigments, being insoluble and non-migratory are generally considered safe to use in toys and food contact applications. They are easily dispersible in plastics.
Applications of Mixed Oxide Pigments
Because of their inertness and outstanding fastness properties, they find outlets in areas where other pigments are no longer satisfactory; such as exterior formulations where a life expectancy of over 10 years is required, even in pale tints, and in engineering plastics processed at very high temperatures.
Cobalt pigments are often used where phthalocyanine pigments would promote warping and stress cracking e.g. HDPE bottle crates and other large mouldings. As more emphasis is placed on the usage of safer pigments for food packaging and children’s toys this class of pigments is often promoted as heavy metal replacements.
Mixed Oxides/Sulphides
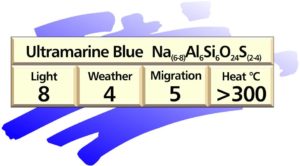
Ultramarine pigments
These pigments, available in a number of shades of blue through to violet, are sulphur containing sodium aluminium silicates. They fall in between mixed oxides and metal sulphides in the general classification.
General formula: Na(6-8)Al6Si6O24S(2-4)
They are produced by a calcination process at about 800ºC from a mixture of china clay, soda, sodium sulphate, sulphur, silica and carbon. Different types are obtained by varying the mixture and thermal conditions.
Properties of ultramarine pigments: The pigment is translucent, with very low hiding power and tinctorial strength. The shade varies from bright, clean reddish blue to violet. Thermal stability is excellent and light and weather fastness is very good. Ultramarine pigments exhibit excellent resistance to most chemicals and solvents, but are attacked by acids which split off hydrogen sulphide and cause fading. Special grades of ultramarines with distinctly improved acid resistance are available.
Ultramarine pigments are easy to disperse and safe to use in toy and food contact applications. The low cost of these pigments is partly offset by their lower hiding power and tinctorial strength.
Applications: because of its excellent resistance to heat, ultramarine blue is used extensively as a blue colourant in plastics and as an additive to correct for yellowness in resins and to produce blue-tone whites.
Metal Sulphides
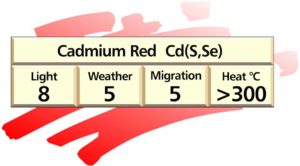
Cadmium pigments
Cadmium pigments are a very versatile group of plastics colorants, and are used in almost all polymers. They are based on the compound cadmium sulphide CdS that produces a golden yellow pigment. Cadmium colours range from primrose yellow to deep maroon. Partial substitution of cadmium by zinc, up to approximately 14% weight/weight gives the lemon and primrose shades. Replacing part of the sulphur with selenium, up to approximately 22% weight/weight results in oranges reds and maroons which can also be obtained by partial substitution of the cadmium with mercury (mercadium pigments).
Cadmium pigments are manufactured by precipitation followed by calcination.
They are first precipitated by mixing together the appropriate salt solutions. The precipitate is washed, dried and then calcined (roasted) at 550ºC to 600ºC to develop the true colours. During calcination the crystal structure changes from cubic to the more stable hexagonal structure. After calcination the pigment is washed with 0.1N HCl to remove more than 99.9% of soluble cadmium, then again washed, dried and disaggregated. The final product is a homogeneous powder with a typical particle size of one micron.
All cadmium pigments are available as the pure pigments and as cadmium lithopones which have been extended with barium sulphate – BaSO4. Cadmium lithopones may be obtained either by co-precipitating with barium sulphate, or by blending the finished full strength pigment with barium sulphate after calcination.
Properties of cadmium pigments
Cadmium pigments are manufactured in a wide range of brilliant opaque colours ranging from primrose yellow to deep maroon. The most important property of cadmium pigments is their excellent heat stability, which makes them almost a mandatory choice in this colour range whenever processing temperatures exceed 300ºC. Cadmium pigments are very lightfast, reds being superior to yellows which may fade if the resin allows moisture penetration. For this reason they perform better in hydrophobic than hydrophilic plastics.
These pigments are resistant to chemical attack. They are inert in plastics and so do not influence the stability and properties of resins. Both the full strength and reduced types are easy to disperse in plastics.
Cadmium pigments feature prominently in the section that deals with toxicology.
Plastics account for roughly 75% of world cadmium pigment consumption. Their choice is virtually mandatory for certain engineering plastics where processing temperatures may reach 400ºC.
Metal Chromates
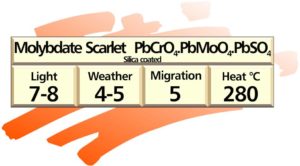
Lead Chromates and Molybdates
These pigments are based on compound lead chromate (PbCrO4) and manufactured by precipitation of the insoluble lead salts from aqueous solution.
Lead chromate is polymorphic (i.e. it can exist in different crystal forms) and it also has the ability to form mixed crystals.
Lead chromate pigments in shades ranging from primrose yellow to scarlet are obtained by co-precipitating lead chromate with lead sulphate and lead molybdate, while adjusting the temperature, pH and concentration of the precipitation bath.
If not after-treated, lead chromate pigments are not very stable and quickly darken and become dull when exposed to heat (approximately 200ºC), light, acids, alkalis, amines, sulphides, etc.
These pigments can be externally stabilised against various influences by enveloping the primary particles with certain oxidic coatings. Silica encapsulated lead pigments have proved most versatile in plastics applications due to their greatly improved heat stability.
Properties of silica-coated lead chromates and molybdates
Lemon yellow to scarlet shades are available possessing good opacity and brightness. Tinctorial strength is high for inorganics. These pigments are heat stable to approximately 300ºC. Light and weather fastness is very good, reds being superior to yellows. Lead chromates darken rather than fade on exposure. They are very easy to disperse and are inert in most polymers.
Because they contain soluble lead they are not recommended for use in children’s toys or plastics intended for food contact.
Applications: Lead chromates and molybdates are very widely used in plastics because of their good balance of properties combined with low cost.
The conventional, less heat stable ones find outlets in LDPE films and PVC, whereas the high heatstable ones are used in most plastics. Their usage is only restricted by toxicological considerations and processing temperatures over 300ºC.
Special effect pigments
There have been many developments in this area, often driven by the cosmetic, decorative and automotive industries.
Aluminium powders
‘Silver’ effect. Produced by reducing the particle size of aluminium chips in ball or hammer mills and coating the fine particles with a suitable lubricant such as stearic acid. Other colours can be obtained by depositing single or multi-layers of metal oxides on the aluminium flakes.
Bronze powders
‘Gold’, ‘bronze’ and ‘copper’ effects. Produced in a similar way to aluminium pigments from brass, bronze and copper chips. Colours range from gold through bronze to metallic copper. They generally tend to darken and dull on exposure to heat. Even the coated, tarnish-resistant grades are inferior to aluminium pigments in this respect.
All metallic pigment tend to highlight flow and weld lines in a moulded article. This affect can be reduced by selecting finer metal particles.
Pearlescent and other special effect pigments
Several compounds can produce a pearlescent effect in plastics. Bismuth oxychloride, lead carbonate and barium thiosulfate have been largely replaced with coated mica particles.
Titanium dioxide coated micas have found the largest acceptance in plastics because of their superior heat stability, non-toxic nature and general inertness. They are available in silver white and coloured grades. Other oxides e.g. iron oxide, tin oxide, chromium oxide, etc. can be used to alter the colours.
Often the colour of the coloured types is produced by interference phenomena. This means that one component of the incident light e.g. green is preferentially reflected, whereas the complementary colour e.g. red is transmitted.
Some very striking special effects marketed under various names like chromaflair and chromashift (not all of them inorganic) have become popular.
Various factors such as the clarity of the base resin and background colour, influence the creation of specific colour effects.
Phosphorescent pigments
These pigments glow-in-the-dark, re-emitting absorbed light. Strontium aluminate has largely replaced zinc sulphide because its luminance is roughly 10 times greater. They both produce a greenish yellow glow. Other colours can be obtained by modifying the composition. In plastics they find outlets in safety and novelty applications. Unfortunately phosphorescent pigments require high addition rates which make them quite expensive in use.
© Vibron 2018